Background
In the past 20 years, many clinical trials with pediatric type chemotherapy have improved outcome in adult ALL patients. However, they were selected patients with good performance status, less comorbidity, and tolerance to intensive chemotherapy. In the real-world, many ALL patients did not receive intensive chemotherapy for various reasons especially in elderly patients. Because the aging of the population is increasing remarkably in Japan, it is important to clarify the current overall treatment results and consider new treatment strategies for all ALL patients. Real-world data in ALL including patients not participating in the clinical trials have rarely been reported. Therefore, Japan Adult Leukemia Study Group (JALSG) conducted JALSG ALL-CS-12 study, a prospective clinical observation study for newly diagnosed adult ALL with large number of patients.
Methods
All newly diagnosed ALL patients according to the 2008 edition of WHO classification aged 15 years and older were prospectively registered by JALSG after informed consent. The study was approved by institutional ethical board and conducted in accordance with the Declaration of Helsinki.
Results
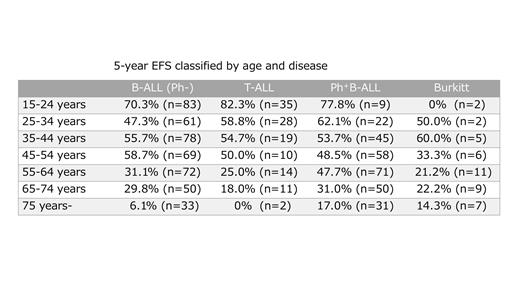
Nine-hundred and fifty-four ALL patients were accrued between April 2012 and August 2017. Nineteen patients were excluded for misdiagnosis including CML blast crisis, history of chemotherapy, lack of data, and duplicate registration. Among the remaining 935 patients, 209 were over 65 years old patients generally not focused on clinical trials. Four-hundred and sixty-nine were Philadelphia chromosome negative B-ALL (Ph-), 130 were T-ALL, 293 were Ph +ALL, and 44 were Burkitt leukemia. Median age was 45 years (range 15-89) in B-ALL (Ph-), 33 (15-80) in T-ALL, 60 (15-89) in Ph +ALL, and 60 (15-91) in Burkitt leukemia with significantly younger in T-ALL patients as has been reported previously. Chemotherapy was not performed in 23 patients in B-ALL (Ph-), 3 in T-ALL, 4 in Ph +ALL, and 3 in Burkitt leukemia. Fifteen of them were over 70 years old. CR rate was comparable in four disease cohorts, 81.7% in B-ALL (Ph-), 78.7% in T-ALL, 88.7% in Ph +ALL and 74.4% in Burkitt leukemia, respectively. However, in the patients with 75 years or older, CR rate was low, 42.4% in B-ALL (Ph-), 64.5% in T-ALL, 64.5% in Ph +ALL and 42.9% in Burkitt leukemia, respectively. Cumulative incidence of relapse at 5 years was 38.8% in B-ALL (Ph-), 20.8% in T-ALL, 43.8% in Ph +ALL, and 50.9% in Burkitt leukemia, respectively (p<0.05). 5-year event free survival (EFS) and overall survival (OS) rates were 46.1% and 52.6% in B-ALL (Ph-), 55.4% and 61.6% in T-ALL, 44.5% and 52.5% in Ph +ALL, and 26.2% and 36.0% in Burkitt leukemia, respectively (p<0.05). When the age of the patients was classified in increments of 10 years, EFS was worse as the age increased in B-ALL (Ph-), T-ALL, and Ph +ALL (table).
Conclusions
JALSG ALL-CS-12 study is the large prospective cohort study and provides important information that is less to the inherent selection bias. The incidence and median age of the four types of leukemia cohorts in Japan were comparable to those in European reports. Although CR rate was acceptable despite the inclusion of patients outside of clinical trials, EFS was strongly dependent on age. Post-remission treatment and management of complications are assignments to be solved especially for elderly ALL patients.
Acknowledgement
We thank Ms. Ryoko Fujiyoshi in Nagasaki data center for collecting CRFs.
Disclosures
Hatta:Kyowa Kirin: Honoraria; Chugai: Honoraria; Ono Pharma: Honoraria; Takeda: Honoraria; Bristol‐Myers Squibb: Honoraria; Janssen Pharma: Honoraria. Murayama:Novartis Pharma K.K.: Honoraria; Takeda Pharmaceutical Company Limited: Honoraria; SymBio Pharmaceuticals Limited: Honoraria; Kyowa Kirin Co.,Ltd.: Honoraria; Ono Pharmaceutical CO. Ltd..: Honoraria; Nippon Shinyaku Co., Ltd: Honoraria; Mundipharma K.K.: Honoraria; Eisai Co., Ltd.: Honoraria; Janssen Pharmaceutical K.K.: Honoraria; Bristol Myers Squibb K. K.: Honoraria; AbbVie GK: Honoraria; Chugai Pharmaceutical Co.Ltd: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Honoraria. Dobashi:Mundipharma K.K.: Research Funding; Kissei Pharmaceutical Co., Ltd.: Research Funding; Novartis Pharma K.K.: Speakers Bureau; Amgen K.K.: Speakers Bureau; Janssen Pharmaceutical K.K.: Speakers Bureau; Nippon Shinyaku Co., Ltd.: Speakers Bureau; AbbVie GK.: Research Funding, Speakers Bureau; Otsuka Pharmaceutical Co., Ltd.: Consultancy, Research Funding, Speakers Bureau; Chugai Pharmaceutical Co., Ltd.: Research Funding, Speakers Bureau; AstraZeneca K.K.: Speakers Bureau; Eisai Co., Ltd.: Speakers Bureau; Kyowa Hakko Kirin Co., Ltd.: Research Funding; Daiichi Sankyo Co., Ltd.: Research Funding; Taiho Phamaceutical Co., Ltd.: Research Funding; Astellas Pharma Inc.: Speakers Bureau. Ota:Amgen: Speakers Bureau; AstraZeneca: Speakers Bureau; Bristol Myers Squibb: Speakers Bureau; Novartis: Speakers Bureau; Janssen: Speakers Bureau. Tanaka:Astellas Phrama: Speakers Bureau; MSD: Speakers Bureau; Asahi Kasei Pharma: Speakers Bureau; Chugai Pharmaceutical: Speakers Bureau; Pfizer: Speakers Bureau; Otsuka Pharmaceutical: Speakers Bureau; Kyowa-Kirin: Speakers Bureau; Sumitomo Pharma: Speakers Bureau; Daiichi Sankyo: Speakers Bureau; Abbvie: Speakers Bureau. Sakaida:Human Life Cord Japan: Consultancy; Ohara: Consultancy; Novartis: Consultancy, Speakers Bureau; Bristol Myers Squibb: Consultancy, Research Funding, Speakers Bureau; Kyowa-Kirin: Research Funding; Otsuka: Consultancy; Takeda: Speakers Bureau; Janssen: Speakers Bureau; Chugai: Research Funding; Pfizer: Consultancy, Speakers Bureau. Maeda:AstraZeneca: Research Funding; Takeda Pharmaceutical Co., Ltd: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria, Research Funding; Astellas: Honoraria, Research Funding; Nippon Shinyaku Pharmaceutical Co., Ltd.: Honoraria, Research Funding; Novartis Pharma: Research Funding, Speakers Bureau; Kyowa Kirin: Honoraria; Eisai Pharmaceutical Co., Ltd.: Honoraria; Otsuka Pharmaceutical: Honoraria. Yamauchi:Abbvie: Honoraria, Research Funding; Chugai: Honoraria, Research Funding; Sumitomo Pharma: Honoraria, Research Funding; Janssen Pharma: Honoraria, Research Funding; Nihon Shinyaku: Honoraria, Research Funding; Astellas: Honoraria, Research Funding; Nihon Kayaku: Honoraria, Research Funding. Matsumura:NIHON PHARMACEUTICAL CO.LTD.: Research Funding; ONO PHARMACEUTICAL CO., LTD.: Honoraria, Research Funding; TAIHO PHARMA: Research Funding; Bristol-Myers Squibb Company: Honoraria; Eisai Co., Ltd.: Research Funding; Genmab K.K.: Research Funding; Chugai Pharmaceutical Co., Ltd.: Research Funding; Kyowa Kirin: Research Funding; Alexion Pharmaceuticals, Inc.: Research Funding; Novartis Japan: Honoraria, Research Funding; Otsuka Pharmaceutical Co., Ltd.: Honoraria; AbbVie GK.: Honoraria, Research Funding; AstraZeneca K.K.: Honoraria; SymBio Pharmaceuticals: Honoraria; Pfizer Japan Inc.: Honoraria, Research Funding; Janssen Pharmaceutical K.K.: Honoraria; Sumitomo Pharma Co., Ltd.: Research Funding; Asahi Kasei Pharma Corporation.: Research Funding; NIPPON SHINYAKU CO., LTD.: Research Funding; Takeda Pharmaceutical Company Limited: Research Funding; SHIONOGI & CO., LTD.: Research Funding. Miyazaki:Kyowa-Kirin: Honoraria; Novartis: Honoraria; Celgene: Honoraria; Nipponshinnyaku: Honoraria; Chugai: Honoraria; Otsuka: Honoraria; Astellas: Honoraria; Dainippon-Sumitomo: Honoraria. Kiyoi:Nippon Kayaku: Honoraria; Amgen: Honoraria; Ono Pharmaceuticals: Honoraria; AstraZeneca: Honoraria; Meiji Seika: Honoraria; SymBio: Honoraria; Asahi Kasei: Research Funding; Eisai: Honoraria, Research Funding; Sumitomo Dainippon Pharma: Research Funding; Zenyaku Kogyo: Research Funding; Bristol-Myers Squibb: Honoraria; Novartis: Honoraria; Chugai: Honoraria, Research Funding; Astellas Pharma: Honoraria, Research Funding; CURED: Research Funding; AbbVie: Honoraria, Research Funding; Daiichi Sankyo: Honoraria, Research Funding; Perseus Proteomics: Research Funding; Otsuka Pharmaceutical: Research Funding; Kyowa Hakko Kirin: Research Funding; Pfizer: Honoraria; Towa: Honoraria; MSD: Honoraria; Pharma Essentia Japan: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal